 |
|
 |
Room temperature ionic liquids |
Room temperature ionic liquids (RTIL) are a class of organic salts with unusually low melting points that attracted
much attention from the scientific community in the recent years. Apart from being liquid at room temperature, ionic liquids
exhibit a negligible vapor pressure and interesting solvation or coordination properties that depend on the characteristics of
the cation and anion. They consists of a 1-alkyl-3-methylimidazolium cation and various inorganic anions like
hexafluorophosphate or tetrafluoroborate. 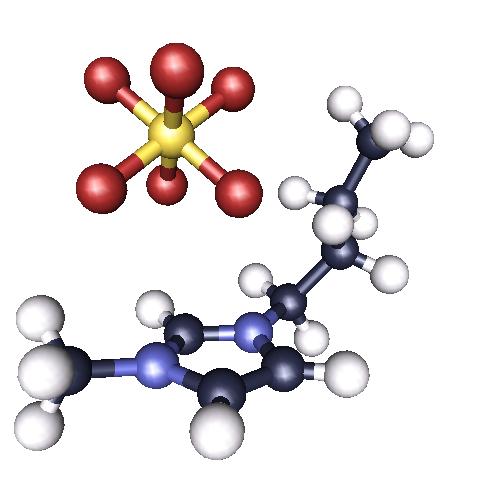 Their status as green or designer solvents explains the large amount of studies concerning
their possible industrial use as reaction or extraction media.
Their status as green or designer solvents explains the large amount of studies concerning
their possible industrial use as reaction or extraction media.
Among other things we plan to use molecular dynamics to study the liquid structure in association with dielectric reflection
spectroscopy, investigate dynamic and electrostatic properties and study solvation of various proteins.
The major result from the experiment is the existence of a broad dielectric dispersion/absorption region in the MHz/GHz regime.
Intuitively one would expect the dielectric functions in this regime to be determined by dipolar reorientations of the ions
and/or ion pairs, because the structures of the cations and anions indicate appreciable dipole moments, and ion pairs are
expected to have very high dipole moments as well. The results give a first impression of what could be expected for the
diffusive response that may govern the long-time behavior of experiments at higher frequencies, but are yet little understood
in detail.
|
|

