The photodissociation and recombination of iodine is one of the most extensively studied
chemical reactions in liquids and, therefore, served as a model system for understanding
various aspects of condensed phase reaction dynamics.
The interest goes back to Franck and Rabinowitch proposing the possibility that after
photoexcitation separating iodine atoms could be trapped in a solvent cage reducing the
dissociation quantum yield with respect to the gas phase.
After light absorption in the range 450 - 600 nm the dissociative B"1u or the
predissociative B0u+ electronic state is populated causing the iodine atoms to seperate
on repulsive potentials. A fraction of atoms collide with surrounding solvent particles and recombine
geminately in the cage. Partially they get trapped in weakly bound A1u and A'2u electronic states from
which they can relax to the ground state. Partially they immediately recombine to the ground state
where the iodine molecules are stabilised by vibrational energy relaxation. A fraction of atoms
succeeds to escape from the solvent cage. These atoms call still recombine geminately within several
tens of picoseconds or separate by diffusion and finally recombine nongeminately on a microsecond time
scale.
The dynamics is characterised by a variety of competing processes summarised in Fig.1.
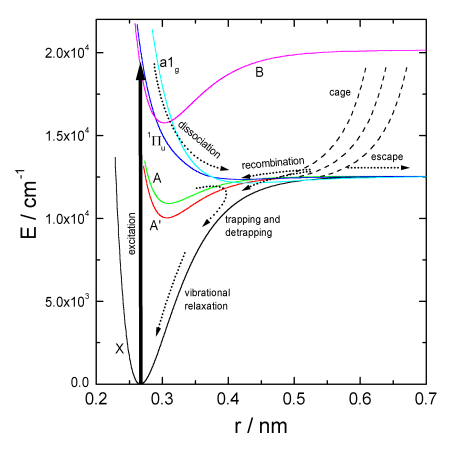
Fig.1. Potential energy curves of iodine
We investigated the geminate recombination dynamics of iodine by determing photodissociation quantum
yields after laser excitation at 532 nm in compressed liquid n-alkanes and supercritical CO2
and xenon. For all solvent the quantum yield was found to decay nearly linearly with increasing density.
The quantum yield is independent to the nature of the solvent but determined by the free space available to
the separating iodine atoms during the dissociation process (Fig.2.). Our results are supported by molecular
dynamics simulations and clearly show that the geminate recombination of iodine can be understood in terms
of a purely kinematic effect.
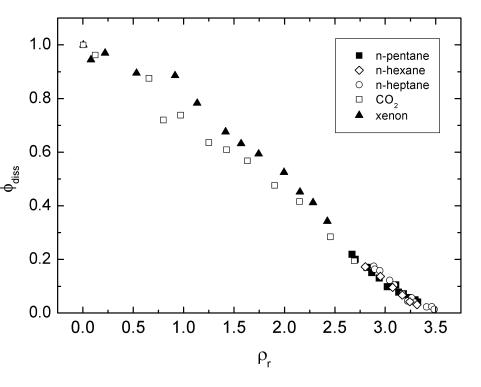 Fig.2. Quantum yields of iodine in various solvents.
Fig.2. Quantum yields of iodine in various solvents.
Literature :
Zeitschrift für Physikalische Chemie, 215, 2, 183-195 (2001)
| 
